Research Interests
Catalysis for Renewable Fuels & Carbon Capture
|
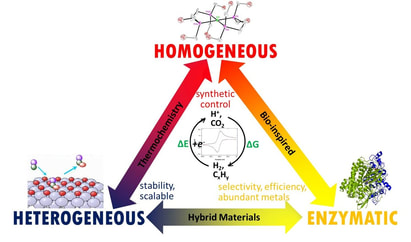
Our research is centered on developing efficient and abundant metal catalysts for the production of fuels or feedstock chemicals using renewable energy. Our approach takes inspiration from enzymatic active sites as well as thermochemical activity descriptors used in heterogeneous catalysis. The initial targets are the electrocatalytic reduction of water to hydrogen, and carbon dioxide to more energy-dense carbon fuels.
The research combines synthesis with advanced electrochemical and spectroscopic techniques. Detailed mechanistic and kinetic studies are employed to improve catalyst design and optimize activity. A public lecture on our research motivation can be found here: https://www.youtube.com/watch?v=mp0hTyIUfPM |
Aqueous Hydrogen and Formate Production
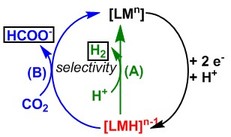
Transition metal hydrides (shown in red to the left) are critical intermediates in hydrogen production (path A) and carbon dioxide reduction to formate (path B). In order to improve our understanding of electrochemical hydride generation and reactivity, we are forming predictive models of hydricity in aqueous solvents. We are also investigating if hydricity is an appropriate activity descriptor for catalytic activity. Additionally, quantifying the hydricity will facilitate the design of catalysts with tailored product selectivity.
To date, we have developed a new complex for electrocatalytic aqueous hydrogen production and an abundant transition metal hydride complex that can perform hydride transfer to carbon dioxide.
Relevant Publications:
To date, we have developed a new complex for electrocatalytic aqueous hydrogen production and an abundant transition metal hydride complex that can perform hydride transfer to carbon dioxide.
Relevant Publications:
- "Translating Aqueous CO2 Hydrogenation Activity to Electrocatalytic Reduction with a Homogeneous Cobalt Catalyst"
Wang, X. S.; Yang, J. Y.*, Chem. Commun., 2023, 59, 338-341. DOI: 10.1039/D2CC05473F - "Thermochemical Studies of Nickel Hydride Complexes with Cationic Ligands in Aqueous and Organic Solvents"
Cypcar, A.; Kerr, T.; Yang, J. Y.* Organometallics, 2022, 41(18), 2605–2611. - "Reducing CO2 to HCO2- at Mild Potentials: Lessons from Formate Dehydrogenase"
Yang, Jenny Y.*; Kerr, Tyler; Wang, Xinran S.; Barlow, Jeffrey M., J. Am. Chem. Soc., 2020, 142(46), 19438–19445 - "Kinetic and Mechanistic Analysis of a Synthetic Reversible CO2/HCO2- Electrocatalyst"
Cunningham, Drew C.; Yang, J. Y.*, Chem. Commun., 2020, 56,12965 - 12968 - "Reversible and Selective CO2 to HCO2- Electrocatalysis near the Thermodynamic Potential"
Cunningham, D. W.; Barlow, J. M.; Velasquez, R. S.; Yang, J. Y.*, Angew. Chemie. Int. Ed., 2020, 59(11), 4443-4447. - "Highly Selective Electrocatalytic CO2 Reduction by [Pt(dmpe)2]2+ through Kinetic and Thermodynamic Control"
Ceballos, Bianca M.; Yang, J. Y.* Organometallics, 2020, 39(9), 1491–1496. - "Directing the reactivity of metal hydrides for selective CO2 reduction" (pdf) Ceballos, B. M.; Yang, J. Y.* Proc. Natl. Acad. Sci., 2018, 115(50), 12686 - 12691.
- "pH-Dependent Reactivity of a Water-Soluble Nickel Complex: Hydrogen Evolution vs Selective Electrochemical Hydride Generation" (pdf). Tsay, C.; Ceballos, B. M.; Yang, J. Y.* Organometallics, 2019, 38(6), 1286-1291.
- "CO2 Reduction or HCO2- Oxidation? Solvent Dependent Thermochemistry of a Nickel Hydride Complex" (pdf). Ceballos, Bianca M.; Tsay, C.; Yang, J. Y.* Chem. Commun. 2017, 53, 7405-7408.
- "Electrocatalytic Hydrogen Evolution under Acidic Aqueous Conditions and Mechanistic Studies of a Highly Stable Molecular Catalyst" (pdf). Tsay, C.; Yang J. Y.*, J. Am. Chem. Soc., 2016, 138(43), 14174-14177.
- "Solvation Effects on Transition Metal Hydricity" (pdf). Tsay, C.; Livesay, B.^; Ruelas, S.^; Yang, J. Y.*, J. Am. Chem. Soc. 2015, 137(44), 14114-14121.
Proximal Cation-induced Electric Field Effects on Reactivity

The active site of the Ch Ni-CODH II enzyme suggests carbon dioxide activation occurs through a cooperative interaction between a Lewis basic nickel and a Lewis acidic iron (shown on the left). We are developing synthetic mimics that position a Lewis acid proximate to a Lewis basic metal center in order to replicate this cooperative interaction. Our initial studies found proximal redox-inactive cations to redox active metals can significantly tune the redox potential of the latter. We have found evidence the shifts in redox potential are likely due to an electric field potential from the cation. We are also exploring unusual trends in redox reactivity and catalysis by proximal cations.
Relevant Publications:
Relevant Publications:
- "Charge and Solvent Effects on the Redox Behavior of Vanadyl Salen-Crown Complexes"
- Nguyen, H. M.; Morgan, H. W. T.; Chantarojsiri, T.; Kerr, T. A.; Yang, J. Y.; Alexandrova, A. N.*; Leonard, N. G.* J. Phys. Chem. A, 2023, 127(25), 5324-5334.
- "Cationic Effects on the Effective Hydrogen Atom Bond Dissociation Free Energy of High Valent Manganese Imido Complexes"
- Leonard, N.; Chantarojsiri, T.; Ziller, J.; Yang, J. Y.*, J. Am. Chem. Soc., 2022, 144(4), 1503-1508.
- "Inhibiting the Hydrogen Evolution Reaction (HER) with Proximal Cations: A Strategy for Promoting Selective Electrocatalytic Reduction"
Barlow, J. M.; Ziller, J. W.; Yang, J. Y., ACS Catalysis, 2021, 11, 8155-8164. - "Synthesis and Redox Properties of Heterobimetallic Re(bpyCrown-M1)(CO)3Cl Complexes, where M1 = Na+, K+, Ca2+, and Ba2+"
Idris, N. S.†; Barlow, J. M.†; Chabolla, S. A.; Ziller, J. W.; Yang, J. Y.*, Polyhedron, 2021, 208, 115385. - "Electric Fields in Catalysis: From Enzymes to Molecular Catalysts"
- Leonard, N. G.;† Dhaoui, R.;† Chantarojsiri, T.; Yang, J.Y.*, ACS Catalysis, 2021, 11, 10923-10932.
- "Installation of Internal Electric Fields by Non-Redox Active Cations in Transition Metal Complexes"
Kang, K.; Fuller III, J.; Reath, A. H.; Ziller, J. W.; Alexandrova, A. N.*; Yang, J. Y.*, Chem. Sci. 2019, 10, 10135 - 10142. - "Cationic Charges Lead to Inverse Free Energy Relationship for N—N Bond Formation by Mn(VI) Nitrides" (pdf). Chantarojsiri, T.; Reath, A. H.; Yang, J. Y.* Angew. Chem. Int. Ed., 2018, 57, 14037-14042.
- "Incorporation of Redox-Inactive Cations Promotes Iron Catalyzed Aerobic C–H Oxidation at Mild Potentials" (pdf). Chantarojsiri, T.; Ziller, J. W.; Yang, J. Y.* Chem. Sci., 2018, 9, 2567 - 2574.
- "Redox Potential and Electronic Structure Effects of Proximal Nonredox Active Cations in Cobalt Schiff Base Complexes" (pdf). Reath, A. J.; Ziller, J. W.; Tsay, C.; Ryan, A. J.; Yang, J. Y.* Inorg. Chem. 2017, 56(6), 3713-3718.
- "Reactivity of a Series of Isostructural Cobalt Pincer Complexes with CO2, CO, and H+" (pdf). Shaffer, D. W.; Johnson, S. I.; Rheingold, A.; Ziller, J.; Goddard III, W.; Nielsen, R. J.; Yang, J. Y.*, Inorg. Chem. 2014, 53(24), 13031-13041.
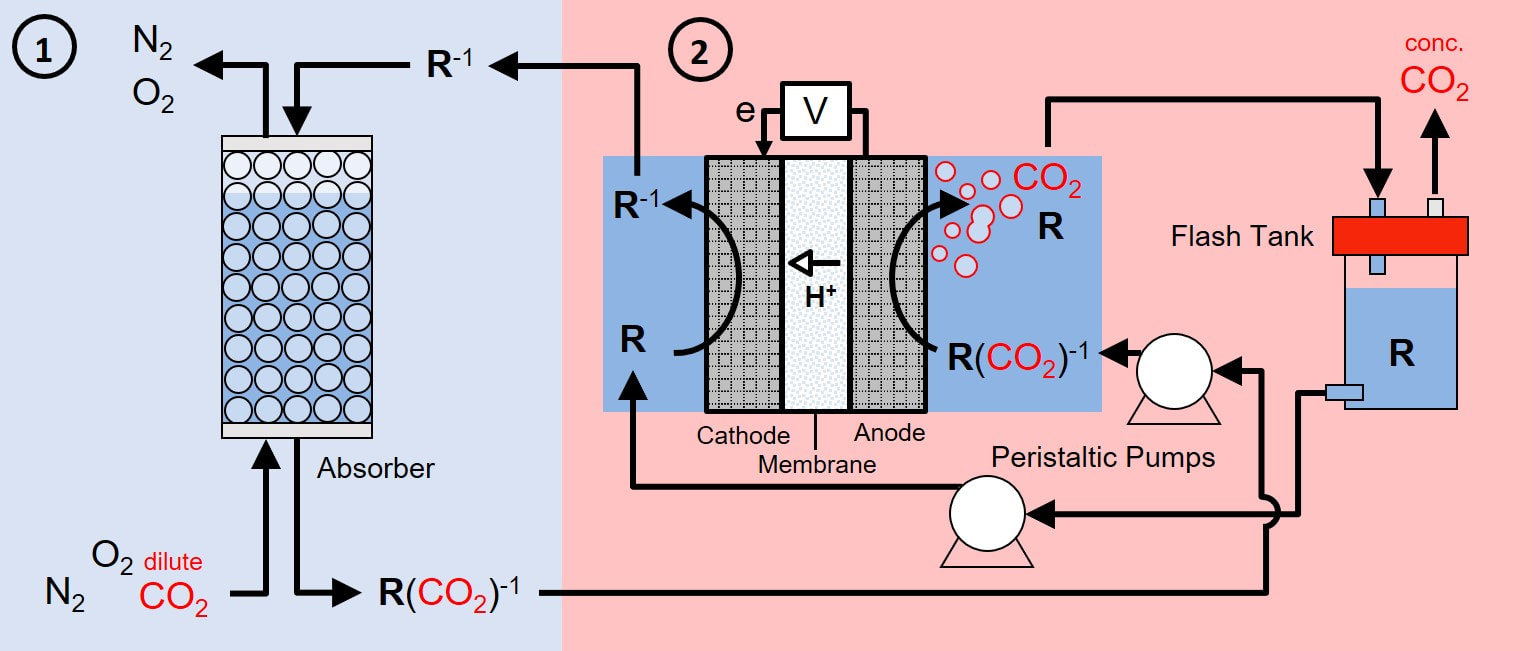
Electrochemical CO2 Capture and Concentration
This project is in collaboration with Prof. Fikile Brushett (MIT) and Prof. Anastassia Alexandrova (UCLA) and is funded by the Sloan Foundation. See press releases from UCI here and from the Sloan Foundation here.
Relevant Publications:
"Electrolyte Effects on the Reduction Potential and Carbon Dioxide Binding Affinity of Quinones"
Zito, A.; Yang, J. Y.*, J. Electrochem. Soc. 2024, 171, 043502
"Aqueous Electrochemical and pH Studies of Redox-Active Guanidino Functionalized Aromatics for CO2 Capture"
Li, C.; Ziller, J.; Barlow, J. M.; Yang, J. Y.*, ACS Organic and Inorganic Au, 2024, accepted.
"Electrochemical CO2 Capture and Concentration"
Zito, A.†; Clarke, L.†; Barlow, J.†; Bim, D.†; Zhang, Z.; Ripley, K.; Li, C.; Kummeth, A.; Leonard, M.; Alexandrova, A.*; Brushett, F.*; Yang, J.Y.*, Chem. Rev., 2023, 123(13), 8069-8098.
"Oxygen Stable Electrochemical CO2 Capture and Concentration through Alcohol Additives"
Barlow, J. M.; Yang, J. Y.*, J. Am. Chem. Soc., 2022, 144(31), 14161–14169
"Computational and Experimental Design of Quinones for Electrochemical CO2 Capture and Concentration"
Zito, A.†; Bim, D.†; Vargas, S.; Alexandrova, A. N.*; Yang, J.Y.*, ACS Sustain. Chem. Eng., 2022, 10(34), 11387–11395.
"Molecular Design of Redox Carriers for Electrochemical CO2 Capture and Concentration"
Barlow, J. M.†; Clarke, L.†; Zhang, Z.†; Bím, D.; Ripley, K., Zito, Z.1, Brushett, F.*, Alexandrova, A. N.*; Yang, J. Y.*, Chem. Soc. Rev., 2022, 51, 8415 - 8433
"Inverse Molecular Design of Alkoxides and Phenoxides for Aqueous Direct Air Capture of CO2"
Zhang, Z.; Kummeth, A.; Yang, J. Y.*; Alexandrova, A. N.*, Proc. Natl. Acad. Sci., 2022,119(25), e2123496119
"Electrolyte Effects on the Reduction Potential and Carbon Dioxide Binding Affinity of Quinones"
Zito, A.; Yang, J. Y.*, J. Electrochem. Soc. 2024, 171, 043502
"Aqueous Electrochemical and pH Studies of Redox-Active Guanidino Functionalized Aromatics for CO2 Capture"
Li, C.; Ziller, J.; Barlow, J. M.; Yang, J. Y.*, ACS Organic and Inorganic Au, 2024, accepted.
"Electrochemical CO2 Capture and Concentration"
Zito, A.†; Clarke, L.†; Barlow, J.†; Bim, D.†; Zhang, Z.; Ripley, K.; Li, C.; Kummeth, A.; Leonard, M.; Alexandrova, A.*; Brushett, F.*; Yang, J.Y.*, Chem. Rev., 2023, 123(13), 8069-8098.
"Oxygen Stable Electrochemical CO2 Capture and Concentration through Alcohol Additives"
Barlow, J. M.; Yang, J. Y.*, J. Am. Chem. Soc., 2022, 144(31), 14161–14169
"Computational and Experimental Design of Quinones for Electrochemical CO2 Capture and Concentration"
Zito, A.†; Bim, D.†; Vargas, S.; Alexandrova, A. N.*; Yang, J.Y.*, ACS Sustain. Chem. Eng., 2022, 10(34), 11387–11395.
"Molecular Design of Redox Carriers for Electrochemical CO2 Capture and Concentration"
Barlow, J. M.†; Clarke, L.†; Zhang, Z.†; Bím, D.; Ripley, K., Zito, Z.1, Brushett, F.*, Alexandrova, A. N.*; Yang, J. Y.*, Chem. Soc. Rev., 2022, 51, 8415 - 8433
"Inverse Molecular Design of Alkoxides and Phenoxides for Aqueous Direct Air Capture of CO2"
Zhang, Z.; Kummeth, A.; Yang, J. Y.*; Alexandrova, A. N.*, Proc. Natl. Acad. Sci., 2022,119(25), e2123496119
Ligand Development
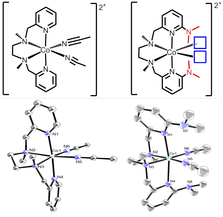
Small molecule oxidation and reduction chemistry often includes proton transfer events. Uncoupled proton transfer can impede catalytic rate or result in high activation barriers for catalysis. To facilitate proton transfer, we are designing new macrocyclic ligands that incorporate pendant acids or bases in the secondary coordination sphere. Recently, we modified the diamine dipyridyl ligand shown on the left with dimethyl pendant amines (red) in the secondary coordination sphere. The blue boxes represent open coordination sites on the Co(II) complex which are occupied by acetonitrile ligands in the X-ray crystal structure. We are currently investigating the small molecule reactivity and redox chemistry of these complexes.
Relevant Publications:
Relevant Publications:
- "Modular Preparation of Cationic Bipyridines and Azaarenes via C–H Activation"
King, R. P.; Yang, J. Y.*, Chem. Sci, 2024, 14, 13530 - 13536 - "Intramolecular Hydrogen-Bonding in a Cobalt Aqua Complex and Electrochemical Water Oxidation Activity" (pdf). Kotyk, J. F. K.; Hanna,C. M.; Combs, R. L.^; Ziller, J. W.; Yang, J. Y.* Chem. Sci. 2018, 9, 2750 - 2755.
- "Copper Tetradentate N2Py2 Complexes with Pendant Bases in the Secondary Coordination Sphere: Improved Ligand Synthesis and Protonation Studies" (pdf) Kotyk, J. F. K.; Ziller, J. W.; Yang, J. Y.*, J. Coord. Chem., 2016, 69(11-13), 1990-2002.
- "Incorporation of Hydrogen-Bonding Functionalities into the Second Coordination Sphere of Iron-Based Water-Oxidation Catalysts” (pdf). Hoffert, W. A.; Mock, M. T.; Appel, A. M.; Yang, J. Y.* Eur. J. Inorg. Chem. 2013, 22-23, 3846-3857.
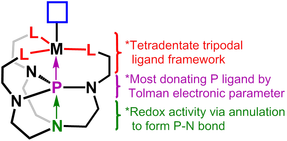
We have also developed a new tripodal tetradentate ligand incorporating a strong phosphine donor based on proazaphosphatrane, shown on the right. The Tolman parameter indicates it is among the most donating phosphines that has been measured. Additionally, the possibility of a transannular interaction in the proazaphosphatrane could increase the donor strength. We are currently investigating the trans effect of the strong phosphine donor on promoting unusual reactivity.
Relevant Publications:
Relevant Publications:
- "Modular Synthesis of Symmetric Proazaphosphatranes Bearing Heteroatom Donors"
Thammavongsy, Z.; Yang, J. Y.* Tetrahedron Letters, 2020, in press. - "Adaptable Ligand Donor Strength: Tracking Transannular Bond Interactions in Tris(2-pyridylmethyl)-azaphosphatrane (TPAP)" (pdf). Zhammavongsy, Z.; Cunningham, D. W.; Sutthirat, N.^; Eisenhart, R. J.; Ziller, J. W.; Yang, J. Y.* Dalton Trans., 2018, 47, 14101-14110.
- "Electronic and Steric Tolman Parameters for Proazaphosphatranes, the Superbase Core of the Tri(pyridylmethyl)azaphosphatrane (TPAP) Ligand" (pdf). Thammavongsy, Z.; Kha, I. M.^; Ziller, J. W.; Yang, J. Y.*, Dalton Trans., 2016, 45, 9853-9859.
- "Flexibility is Key: Synthesis of a Tripyridylamine (TPA) Congener with a Phosphorus Apical Donor and Coordination to Cobalt(II)" (24.pdf). Thammavongsy, Z.; Kotyk, J. F. K.; Tsay, C.; Yang, J. Y.*, Inorg. Chem., 2015, 54(23), 11505-11510.
Integrating Molecular Catalysts onto Photoelectrodes
|
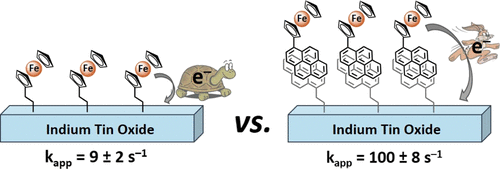
Photoelectrochemical cells offer an integrated path towards direct chemical fuel generation from solar energy. However, most photoabsorbers are poor catalysts and require functional coupling with efficient molecular electrocatalysts. We are developing a milder method of catalyst attachment that is stable, synthetically accessible, and provides facile electron transfer.
|
Relevant Publications:
- "Decoupling Kinetics and Thermodynamics of Interfacial Catalysis at a Chemically Modified Black Silicon Semiconductor Photoelectrode"
Hanna, C. M.; Pekarek, R. T.; Miller, E. M.; Yang, J. Y.*; Neale, N. R.*, ACS Energy Letters 2020, in press. - "Proton-Coupled Electron Transfer at Anthraquinone Modified Indium Tin Oxide Electrodes" (pdf) Hanna, C. M.; Luu, A.; Yang, J. Y.* ACS Appl. Energy Mat. 2019, 2(1), 59-65.
- "Interfacial Electron Transfer of Ferrocene Immobilized onto Indium Tin Oxide through Covalent and Noncovalent Interactions" (pdf). Hanna, C. M.; Sanborn, C. D.; Ardo, S.; Yang, J. Y.* ACS Appl. Mater. Interfaces, 2018, 10(15), 13211-13217.
- "Chemical Modification of Gold Electrodes via Non-Covalent Interactions" (pdf). Lydon, B. L.; Germann, A.^; Yang, J. Y.*, Inorg. Chem. Front., 2016, 3, 836-841.